fda forms|form fda 2252 : Tuguegarao Forms. Official FDA applications and submissions forms. Electronic . Enjoy the hottest furry videos on Rule34video.com, the best site for free furry porn. Watch zonkpunch and other models in action.to continue to Outlook. No account? Create one! Can’t access your account?
fda forms,Reports, Manuals, & Forms. Forms. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Use the following .fda formsU.S. Food and Drug Administration

Forms. Official FDA applications and submissions forms. Electronic .Linkedin. This page provides links to commonly used clinical trial forms .
About FDA. Reports, Manuals, & Forms. Forms. Medical Device Forms.
Instructions for forms; FDA's receipt of the IND Forms: Form FDA 1571 (PDF - .form fda 2252An Investigational New Drug (IND) application is a request for FDA to .Forms. This collection includes forms for applications and submissions, reports .Find out the latest revisions and updates to FDA forms for drug and biologic applications, such as FDA 356h and FDA 1571. Learn how to use the new forms and access the .
Application form is downloaded from www.fda.gov.ph. The integrated application form in XLS or XLSX format is used for both License and Registration applications, as well as .Featured Activity Featured Activities Featured Activity || FDA MEETS WITH OSAPIEA By Administrator 3 / April 1, 2024 On 22 March 2024, the Food and Drug Administration (FDA) led by Director General Dr. Samuel A. .
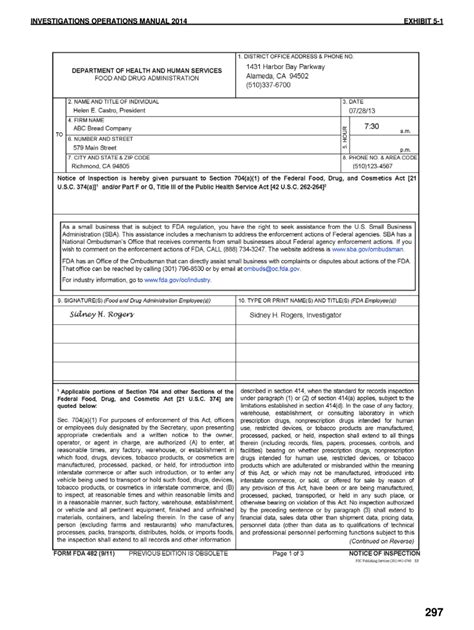
Title: FORM FDA 3674 Author: PSC Publishing Services Subject: Certification of Compliance Under 42 U.S.C. § 282\(j\)\(5\)\(B\), with Requirements of ClinicalTrials.gov Data BankExempt Device Review Form (PDF - 16KB) 510 (k) Cover Sheet Memorandum (PDF - 41KB) 510 (k) "Substantial Equivalence" Decision Making Process (PDF - 844KB) Indications for Use (PDF - 1.7MB .Investigational New Drug Application. Form FDA 1572. Statement of Investigator. Form FDA 3454. Certification: Financial Interests and Arrangements of Clinical Investigators. Form FDA 3455 .New and Updated FDA Forms; Requesting Forms from the Warehouse; Home; About FDA; Reports, Manuals, & Forms; Forms; . Follow FDA on LinkedIn View FDA videos on YouTube Subscribe to FDA RSS feeds. 6. Submit Form FDA 3926** within 15 business days of FDA emergency use authorization. Within 15 business days of FDA emergency use authorization, submit Form FDA 3926 (along with the LOA) to FDA .
Downloadables Public Assistance Information and Receiving Integrated Application Forms and Process In pursuant to FDA Circular No. 2014-003: Filing and Receiving of Registration, Licensing and Other Applications Using the Integrated Application Form, the following are the steps of the LTO and CPR ProcessingDownload. Application form is .U.S. Food and Drug AdministrationAn Investigational New Drug (IND) application is a request for FDA to administer an investigational drug to humans. Sponsors submitting INDs should include Form 1571. For guidance on Form 1571, and to download a fillable PDF, select this link. To apply to market a new drug, biologic, or an antibiotic drug for human use, you need to complete .
New and Updated FDA Forms; Requesting Forms from the Warehouse; Home; About FDA; Reports, Manuals, & Forms; . Follow FDA on LinkedIn View FDA videos on YouTube Subscribe to FDA RSS feeds.
INSTRUCTIONS FOR FILLING OUT FORM FDA 356h – APPLICATION TO MARKET A NEW OR ABBREVIATED NEW DRUG OR BIOLOGIC FOR HUMAN USE. (The field numbers below correspond to the numbered boxes on the Form .U.S. Food and Drug Administration 2022 SPL Xforms Training Sessions. Registration Information. There is no registration fee for the training sessions but pre-registration is required because of limited connections for the webinars .FDA Industry Systems (FIS) was created to facilitate making submissions to the U.S. Food and Drug Administration (FDA), including registrations, listings, and other notifications. . Form 3613, 3613a, 3613c and 3613g OMB Approval Number 0910-0498 OMB Expiration Date 04/30/2024 See OMB Burden Statement. Info Help.Safety reporting portal for health professionals, patients, consumers and industry. Find pdf fillable forms in English and Spanish and a link to report safety concerns to the FDA online.form fda 1571 supplement (0323) – form instructions (previs editins sete) page 1 of 6 psc publishing services (301) 443-6740 ef instructions for filling out form fda 1571
fda forms form fda 2252 At the end of March 2023, the FDA released two new forms that replace previous versions: Form 1571: Investigational New Drug Application (IND) form. Form 356h: Form for New Drug Applications (NDAs) and Biologics License Applications (BLAs) Both forms represent several changes and add additional information to help the FDA’s . Please mail the completed form to FDA’s Registration and Listing of Cosmetic Product Facilities and Products Program at: Food and Drug Administration, Office of Cosmetics and Colors .
Draft FDA Form 5067 - Cosmetic Product Listing Announcements. 8/7/2023 - .
fda forms|form fda 2252
PH0 · form fda 2252
PH1 · fda form 3674
PH2 · fda form 3654
PH3 · fda form 3514
PH4 · fda form 3454
PH5 · Iba pa
PH6 · 356h form fda
PH7 · 356h form